Top 5 Medical Technology Innovations
March 2013
Against the backdrop of health care reform and a controversial medical
device tax, medical technology companies are focusing more than ever on
products that deliver cheaper, faster, more efficient patient care. They
are also making inroads with U.S. Food & Drug Administration
regulators to re-engineer the complex review and approval process for
new medical devices.Many in the industry have long felt overly burdened by what they consider to an unnecessarily complex approval process. Critics claim it impedes innovation and delays the availability of better health care. To change that perception, the FDA last year announced a new Medical Device Innovation Consortium (MDIC) charged with simplifying the process of designing and testing new technologies. With input from industry, government, and other nonprofit organizations, public-private MDIC will prioritize the regulatory science needs of the medical device community and fund projects to streamline the process.
"By sharing and leveraging resources, MDIC may help industry to be better equipped to bring safe and effective medical devices to market more quickly and at a lower cost," says Jeffrey Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health.
As the regulators, politicians, and corporate executives hash out these details, industry engineers and scientists continue to push through new ideas for improving and managing human health. Every year, industry observers like the Cleveland Clinic and the medical device trade press single out their favorite technology trends. These thought leaders agree that today's best technologies strike a balance between reducing the overall cost of medical care and increasing safety and survival rates—and isn't that what health care reform is all about?
Here are five emerging technologies to watch in the year ahead.
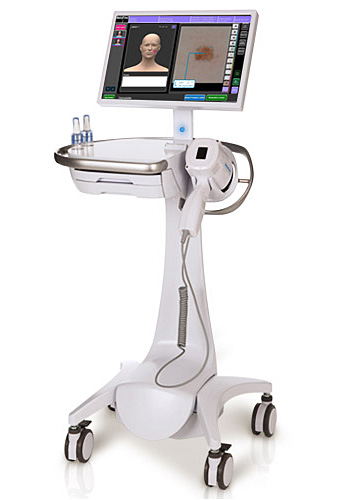
The MelaFind optical scanner from MELA Sciences. Image: MelaFind.com
1. Cutting Back on Melanoma Biopsies
With the most deadly form of skin cancer, melanoma, a huge number of dangerous-looking moles are actually harmless, but has always been impossible to know for sure without an invasive surgical biopsy. Today dermatologists have new help in making the right call — a handheld tool approved by the FDA for multispectral analysis of tissue morphology. The MelaFind optical scanner is not for definitive diagnosis but rather to provide additional information a doctor can use in determining whether or not to order a biopsy. The goal is to reduce the number of patients left with unnecessary biopsy scars, with the added benefit of eliminating the cost of unnecessary procedures. The MelaFind technology (MELA Sciences, Irvington, NY) uses missile navigation technologies originally paid for the Department of Defense to optically scan the surface of a suspicious lesion at 10 electromagnetic wavelengths. The collected signals are processed using heavy-duty algorithms and matched against a registry of 10,000 digital images of melanoma and skin disease.
The ATI Neurostimulator from Autonomic Technologies. Image: ATI-SPG.com
2. Electronic Aspirin
For people who suffer from migraines, cluster headaches, and other causes of chronic, excruciating head or facial pain, the "take two aspirins and call me in the morning" method is useless. Doctors have long associated the most severe, chronic forms of headache with the sphenopalatine ganglion (SPG), a facial nerve bundle, but haven't yet found a treatment that works on the SPG long-term. A technology under clinical investigation at Autonomic Technologies, Inc., (Redwood City, CA) is a patient-powered tool for blocking SPG signals at the first sign of a headache. The system involves the permanent implant of a small nerve stimulating device in the upper gum on the side of the head normally affected by headache. The lead tip of the implant connects with the SPG bundle, and when a patient senses the onset of a headache, he or she places a handheld remote controller on the cheek nearest the implant. The resulting signals stimulate the SPG nerves and block the pain-causing neurotransmitters.
The Symphony tCGM biosensor from Echo Therapeutics. Image: EchoTX.com
3. Needle-Free Diabetes Care
Diabetes self-care is a pain—literally. It brings the constant need to draw blood for glucose testing, the need for daily insulin shots and the heightened risk of infection from all that poking. Continuous glucose monitors and insulin pumps are today's best options for automating most of the complicated daily process of blood sugar management – but they don't completely remove the need for skin pricks and shots. But there's new skin in this game. Echo Therapeutics (Philadelphia, PA) is developing technologies that would replace the poke with a patch. The company is working on a transdermal biosensor that reads blood analytes through the skin without drawing blood. The technology involves a handheld electric-toothbrush-like device that removes just enough top-layer skin cells to put the patient's blood chemistry within signal range of a patch-borne biosensor. The sensor collects one reading per minute and sends the data wirelessly to a remote monitor, triggering audible alarms when levels go out of the patient's optimal range and tracking glucose levels over time.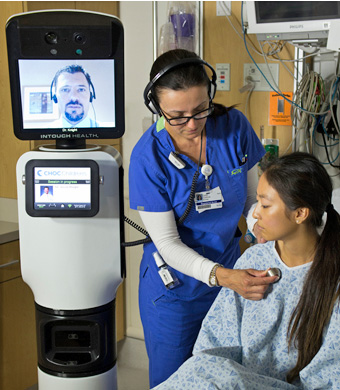
The Telemedicine System from InTouch Technologies. Image: InTouchHealth.com
4. Robotic Check-Ups
A pillar of health reform is improving access to the best health care for more people. Technology is a cost-effective and increasingly potent means to connect clinics in the vast and medically underserved rural regions of the United States with big city medical centers and their specialists. Telemedicine is well established as a tool for triage and assessment in emergencies, but new medical robots go one step further—they can now patrol hospital hallways on more routine rounds, checking on patients in different rooms and managing their individual charts and vital signs without direct human intervention. The RP-VITA Remote Presence Robot produced jointly by iRobot Corp. and InTouch Health is the first such autonomous navigation remote-presence robot to receive FDA clearance for hospital use. The device is a mobile cart with a two-way video screen and medical monitoring equipment, programmed to maneuver through the busy halls of a hospital.
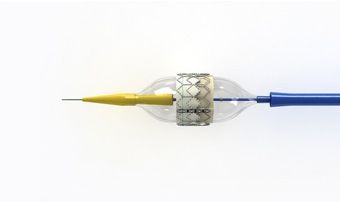 The Sapien transcatheter aortic valve from Edwards Lifesciences. Image: Edwards.com
5. A Valve Job with Heart
The Sapien transcatheter aortic valve from Edwards Lifesciences. Image: Edwards.com
5. A Valve Job with Heart
The Sapien transcatheter aortic valve is a life-saving alternative to
open-heart surgery for patients who need new a new valve but can't
endure the rigors of the operation. Manufactured by Edwards Life Sciences (Irvine, CA), the Sapien
has been available in Europe for some time but is only now finding its
first use in U.S. heart centers—where it is limited only to the frailest
patients thus far. The Sapien valve is guided through the femoral
artery by catheter from a small incision near the grown or rib cage. The
valve material is made of bovine tissue attached to a stainless-steel
stent, which is expanded by inflating a small balloon when correctly
placed in the valve space. A simpler procedure that promises
dramatically shorter hospitalizations is bound to have a positive effect
on the cost of care.
The Sapien transcatheter aortic valve from Edwards Lifesciences. Image: Edwards.com







0 comments:
Post a Comment